This site provides INTERCEPT product information for International audiences Select your region
No Sepsis Fatalities with INTERCEPT Platelets
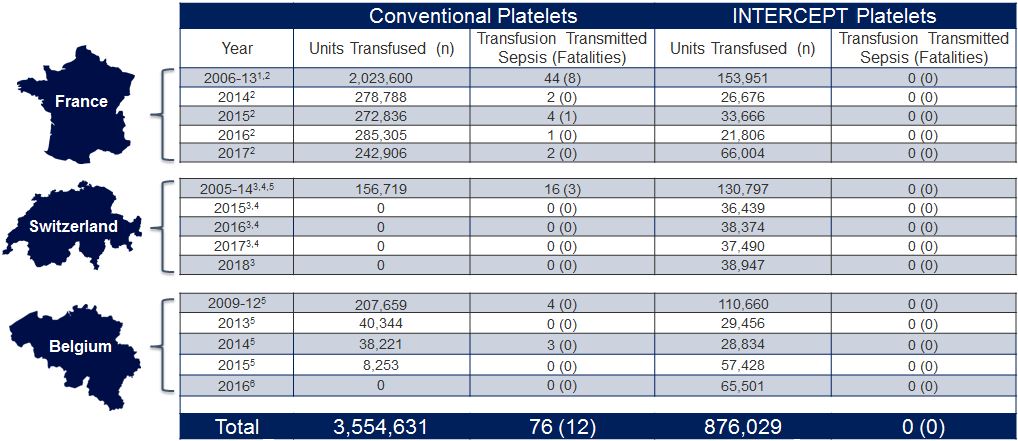
French, Swiss and Belgium National Hemovigilance Data
1Cazenave, JP, et al. Pathogen Inactivation of Platelets. AABB Press: Bethesda, MD 2013; 19-176.
2French National Agency for Medicine and Health Product Safety/ANSM, Hemovigilance Activity Reports, 2009–2018.
3SwissMedic Haemovigilance Annual Reports, 2005–2018.
4Jutzi M. et al. Transfus Med Hemother 2018;45:151-6.
5Benjamin et al. Transfusion 2017;57:2946-57
6AFMPS Hémovigilance Rapport annuel: Belgium. 2016
Transfusion-Associated Bacterial Sepsis Becomes History
Bacterial contamination of platelet concentrates is the most significant infectious risk in transfusion today, despite the introduction of early bacterial culture detection.
The INTERCEPT™ Blood System for platelets achieves effective inactivation for a wide range of bacteria with varying growth rates, including:
- Organisms with a prolonged lag phase or slow growth that may otherwise escape culture detection, such as S. epidermidis and S. aureus 2-4
- Fast-growing bacteria that may rapidly reach high concentrations in platelets, including E. coli, K. pneumonia and S. marcescens 2-4
Since the introduction of the INTERCEPT Blood System for platelets (2005 in France, and 2009 in Switzerland and Belgium), no cases of bacterial sepsis or fatalities have been reported, as demonstrated by French, Swiss and Belgium national haemovigilance data.
Effective pathogen inactivation of bacteria. (create link with table we need to create which contains subset of pathogen inactivation claims for pathogens tested in the blood centre)
Since the pathogen inactivation (PI) process has been implemented for all platelet concentrates (PC) in Switzerland, no additional cases of sepsis due to PC have been reported.
Swissmedic Annual Haemovigilance Report, 2014
Sources:
2.Lin L et al. Transfusion 2004;44:1496-1504.
4.Schmidt M et al. Vox Sang 2001;101(S1):226.

